A photon is a tiny particle of light. It is the tiniest particle of light possible in nature. A photon can also be described as a type of quantum, that is, a tiny particle. Some other types of quanta (plural) are electrons, neutrinos, and the Higgs boson.
A quantum is the tiniest particle possible of a particular substance. For example, in the case of an electron, it’s the tiniest particle possible of negatively-charged matter, just as a photon is the tiniest particle possible of light.
A quantum is as small as it gets.
This description is saying something interesting—Nature does not allow us to cut matter and energy into smaller pieces indefinitely. Let’s say that we could cut a rock down into tiny grains of sand, but there were some natural law that a grain of sand is as small as we can get. If this were true, a grain of sand would be a quantum of rock. But, of course, we can cut a grain of sand into ever smaller grains. Only when we get down to the level of electrons and other subatomic particles, does Nature call a halt to the cutting. That’s when we hit the quantum level.

In summary, a photon is the tiniest possible particle of light, a quantum of light. A quantum, on the other hand, is the tiniest possible particle of any substance at the subatomic level and includes, for example, electrons and neutrinos. If this answers your question, no need to read any further. If you want to know more about photons and quanta, read on.
But isn’t light a wave?
To see how light can be divided into photons, it’s necessary to understand a bit more about light. Light travels as a wave. Specifically, it travels as an electromagnetic wave. An electromagnetic wave is an electrical wave and a magnetic wave traveling together and interacting.
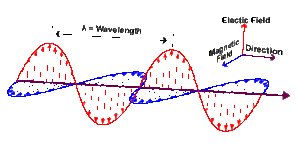
Physicists call all electromagnetic waves “light.” This includes visible light, the kind that we see with our eyes, but also X-rays, ultraviolet rays, infrared, microwaves, radio waves (which carry TV and radio signals), and others. The difference between the various types of electromagnetic waves is their wavelength (shown in image below).
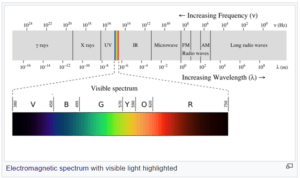
So, light is a wave and also a particle?
When light, that is, an electromagnetic wave, strikes an object, it immediately collapses into tiny bits or particles of energy. (Please don’t take this literally; it’s meant only metaphorically.*) Each of these particles is a photon. It’s as if an ocean wave hits a rock and shatters into a gazillion tiny droplets. Each “droplet” of the light wave is a photon, and each carries a bit of energy. If a wave of visible light were to strike a piece of photographic film, we would be able to see the traces of all the photons which struck it. Each photon creates a tiny dot, a bit of the photo, usually a small fraction of a pixel. Together, the photons form the image.
Waves, including light waves, are spread out in space. When it strikes the film, the light is no longer acting as a wave; it’s acting as a particle. Particles differ from waves in that they are localized, that is, they have a small and definite position.
Photons are the particle form of light.
In summary, light acts as both a wave and a particle. When traveling, it’s an electromagnetic wave. But upon striking objects, it acts as a particle. While “photon” is the name given to light only when it acts as a particle, people may neglect the distinction. They often use the term “photon” for light at all times, whether it’s in wave form or particle form.
As a note, the term “photon” comes from the ancient Greek photos, which means “light” and the ending -on, which means “a particle.” “Photon” means literally “light particle.”
Why call it a “quantum”?
The general term for all types of subatomic particles which are of the smallest possible size allowed by Nature is “quanta.” “Quanta” is the plural; “quantum” is the singular. The term “quantum” comes from the Latin quantus which means “how much.” Electrons are the quanta associated with electron waves; neutrinos are the quanta associated with neutrino waves; etc. Just like photons, electrons cannot be further divided into something smaller, nor can neutrinos.
In its original meaning, a “quantum” is the tiniest particle of a substance that Nature allows. However, often people call any tiny particle that follows the laws of quantum physics a “quantum” even when it’s not the smallest allowed by Nature.
Footnotes
*Light waves don’t physically shatter when they hit objects. They interact with the objects and due to the laws of quantum physics, the waves transform into tiny energy-bearing particles, that is, photons.
The physical nature of waves at the subatomic level is somewhat mysterious. It’s still under debate due, in part, to the odd ways in which these waves behave in experiments. One view, for example, is that they are no more than mathematical expressions in the form of a wave equation which somehow create physical effects (!?). But this is diving deeper into quantum physics than is useful here.
